Calculate the Work Done When 50.0 G of Tin
Calculate the heat absorbed in kJ by the metal. Calculate the change in energy of the gas.
Calculate the work done when 500g of tin dissolves in excess acid at 100atm and 25 degree in celsius Sns2Haq----- Snaq H2g Assume ideal gas behavior The work done pressureincrease in volume The increase in.

. 11871gmol This means 700g tin is500 g11871gmol 042 mol Since one mole Tin reacts to form one mole H2 this means 042 mole H2 will be formed after reaction. The first step in the industrial recovery of zinc from the zinc sulfide ore is roasting that is th. The work done pressure increase in volume The increase in volume is due to the H2 gas created.
A 622-kg piece of copper metal is heated from 205C to 3243C. It is given that 500 g of tin dissolves in excess acid to give 1 mol of hydrogen gas. Assume ideal gas behavior.
A piece of silver of mass 362 g has a heat capacity of 857 JC. The volume of the hydrogen gas produced will be the change in volume of the system due to the chemical reaction. The number of moles n of a given substance is the ratio of the mass of the substance m to the atomic mass M of the element.
Calculate the work done when 500g of tin dissolves in excess acid at 100atm and 25 degree in celsius Sns2Haq----- Snaq H2g Assume ideal gas behavior The work done pressureincrease in volume The increase in volume is due to the H2 gas. Calculate the work done when 500 g of tin dissolves in an excess of acid at 100 atm and 25 o C. As a result 26 J of heat is given off to the surroundings.
Sn s 2 H aq Sn2 aq H2 g Question. A 1800-g sample of phenol C 6 H 5 OH was burned in a bomb calorimeter whose total heat capacity is 1166 kJC. 2 2 1 mol H 1 mol Sn 500 g Sn 0421 mol H 1187 g Sn 1 mol Sn The next step is to find the volume occupied by the hydrogen gas under.
Calculate the work done when 500 g of tin dissolves in excess acid at 100 atm and 25C. Chemistry questions and answers. In this problem whereas to find work and were asked to find work.
Calculate the work done in joules if the gas expands a against a vacuum. Assume that the volume of liquid water is negligible com-. OperatornameSns2 mathrmHa q longrightarrow mathrmSn2a qmathrmH_2g Assume ideal gas behavior.
Sns 1 2H1aq Sn21aq 1 H 2g Assume ideal gas behavior. Sns 2Haq -- Sn2aq H2g Since the molar mass of Sn. Calculate the work done when 500 g of tin dissolves in excess acid at 100 atm and.
Calculate the work done when 500 g of tin dissolves in excess acid at 100 atm and 25C. Sn s 2H aq Sn 2 aq H 2 g 3. Calculate the change in energy of the gas.
Find the number of moles of tin. Sns 2Haq Sn2aq H2g Assume ideal gas behavior. 621 Define these terms.
Calculate the work done when 500 g of tin are dissolved in excess acid at 100 atm and 29C. Assume ideal gas behavior. Calculate the work done when 500 g of tin are dissolved in excess acid at 100 atm and 29C.
PV nRT 0428314297 1040 J 104 kJ. Calculate the work done when 500 g of tin dissolves in excess acid at 100 atm and 25 degrees C. Calculate the work done when 500g of tin dissolves in excess acid at 100atm and 25 degree in celsius Sn s2H aq----- Sn aq H2 g Assume ideal gas behavior.
619 Calculate the work done when 500 g of tin dissolves in excess acid at 100 atm and 25C. That is also the number of moles of H2 that are created. Calculate the work done when 500 g of tin dissolves in excess acid at 100 atm and 25C.
619 Calculate the work done when 500 g of tin dissolves in excess acid at 100 atm and 25 C. What is the specific heat of silver. Sn s 2H aq Sn 2 aq H 2 g Assume ideal gas behavior.
Sn s 2H aq Sn 2 aq H 2 g We first find the number of moles of hydrogen gas formed in the reaction. Thus the work done due to the expansion of H2 gas is. Step 1 of 3.
Im trying to calculate the work done when 500 g of tin dissolves in excess acid at 100 atm and 29815 K texSns2Haq--Sn2aqH_2gtex Here is what Ive done. 50 g of tin Sn is 0421 moles. Answer to Calculate the work done when 500 g of tin dissolves in excess acid at 100 atm and 25C.
Calculate the work done when 500 g of tin dissolves in excess acid at 100 atm and 25C. Calculate the work done when 500 g of tin dissolves in excess acid at 100 atm and 24C. Science Physics Work in.
When were given 500 grams of 10 and our temperature is given as 25 degrees Celsius and our pressure is given at 10 zero atmospheres. What is the specific heat of silv. In chemistry work is found from a formula.
A piece of silver of mass 362 g has a heat capacity of 857 JC. Calculate the work done when 255 g of tin dissolves in excess acid at 110 atm and 22C. Assume ideal gas behaviorSns 2 Haq Sn2aq H2g Question.
Sn s 2H aq Sn 2 aq H 2 g Assume ideal gas behavior. Mass 500 grams water s water 418 Jg C. 618 The work done to compress a gas is 74 J.
Consider a process in which an ideal gas is compressed to one-sixth of its. Calculate the work done when 500 mathrmg of tin dissolves in excess acid at 100 mathrmatm and 25circ mathrmC. Calculate the work done when 500 g of tin dissolves in excess acid at 100 atm and 25C.
620 Calculate the work done in joules when 10 mole of water vaporizes at 10 atm and 100C. Sn s2H aq Sn 2 aq H_2 g Assume ideal gas behavior.

Calculate The Work Done When 50 G Of Iron Is Dissolved In Hcl At 25 C In I A Clo Youtube
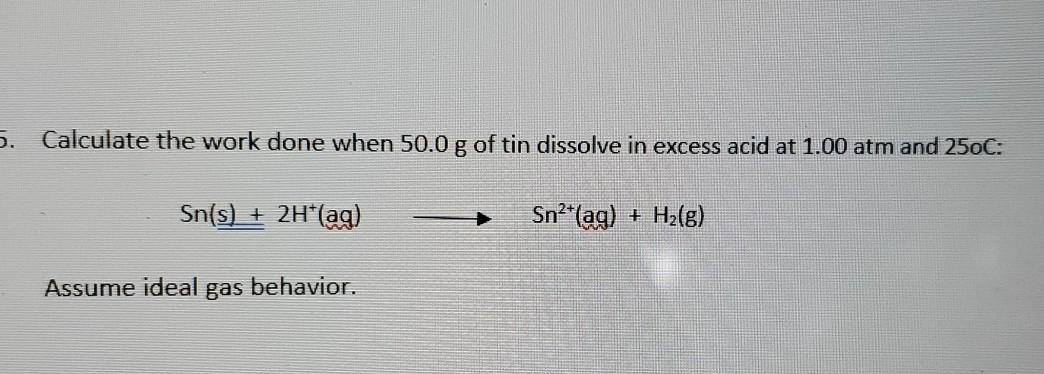
Solved 5 Calculate The Work Done When 50 0 G Of Tin Chegg Com

Solved 50 0 G Of Tin Ii Fluoride Reacts With 50 0 G Of Chegg Com
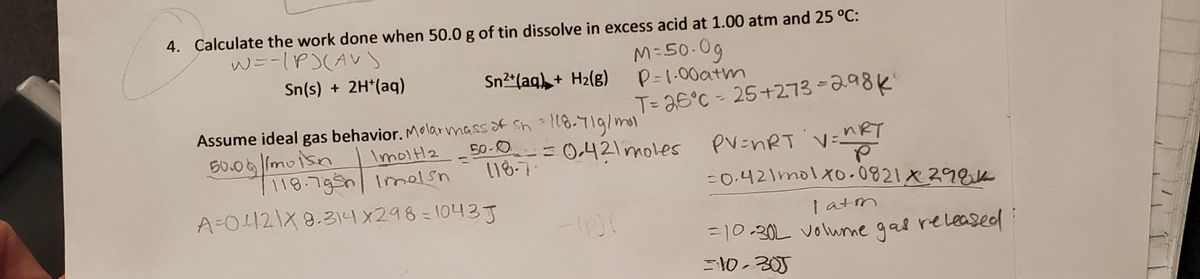
0 Response to "Calculate the Work Done When 50.0 G of Tin"
Post a Comment